


| MOQ: | 10kg |
| Price: | US $500/kg |
| Standard Packaging: | Cylinder/Tank |
| Delivery Period: | 30 days |
| Payment Method: | L/C, T/T |
| Supply Capacity: | 2000 Tons/Year |
Silicon tetrachloride (SiCl4) is a chemical compound composed of one silicon atom bonded to four chlorine atoms. It is a colorless liquid at room temperature, but it can also exist as a volatile gas. Here are some key points about silicon tetrachloride:
Chemical Composition: Silicon tetrachloride consists of one silicon (Si) atom bonded to four chlorine (Cl) atoms. Its chemical formula is SiCl4.
Properties: Silicon tetrachloride is a volatile liquid with a strong, pungent odor. It has a boiling point of 57.6 degrees Celsius (135.7 degrees Fahrenheit) and a melting point of -68.8 degrees Celsius (-91.8 degrees Fahrenheit). It is highly reactive and readily hydrolyzes in the presence of moisture, forming silicic acid and hydrogen chloride.
Production: Silicon tetrachloride is commonly produced through the reaction of silicon dioxide (SiO2) with carbon and chlorine gas:
SiO2 + 2C + 2Cl2 → SiCl4 + 2CO
This reaction occurs at high temperatures in the presence of a carbon-based reducing agent, such as carbon or coke.
Uses: Silicon tetrachloride has various industrial applications:
Silicon Production: It is a crucial precursor in the production of elemental silicon. Silicon tetrachloride is reacted with a reducing agent, such as magnesium, to obtain purified silicon in the form of crystalline or amorphous silicon.
Chemical Synthesis: Silicon tetrachloride is used as a starting material or intermediate in the synthesis of various silicon compounds, including silicones, silanes, and silicon carbide.
Optical Fiber Manufacturing: It is used in the production of high-purity silica glass, which is a key component in optical fibers for telecommunications.
Water Repellents: Silicon tetrachloride is used as a water-repellent treatment for fabrics, paper, and other materials.
Catalysts: It can be employed as a catalyst in certain chemical reactions, particularly in the production of polymers.
Safety Considerations: Silicon tetrachloride is toxic and corrosive. It can cause severe skin burns and eye damage upon contact. Inhalation of its vapors or fumes can be harmful to the respiratory system. Additionally, silicon tetrachloride reacts violently with water, releasing hydrogen chloride gas, which is highly corrosive and toxic. Proper safety precautions, such as the use of protective equipment and appropriate handling procedures, should be followed when working with silicon tetrachloride.
It's important to handle silicon tetrachloride with care and adhere to safety measures to mitigate potential risks associated with its corrosiveness and reactivity.
Basic Info.| Model No: | SiCl4 | Transport Package | ISO Tank |
| Specification | Standard | Trademark | CMC |
| Origin | Suzhou, China | HS Code | 2812190091 |
| Production Capacity | 1000t/Year |
Specification:
| CERTIFICATE OF ANALYSIS | |
| Product Name:Silicon Tetrachloride (STC) | |
| Inspection Item | Inspection Result |
| Product Appearance | Colorless Clear Liquid |
| HCL% | ------ |
| SiH2Cl2% | ------ |
| SiHCl3% | <0.1 |
| SiCl4% | >99.8 |
| High Boiling% | ------- |
| Al | <0.5ppm |
| Fe | <0.5ppm |
| Ti | <0.5ppm |
| Exogenous Impurities | ------ |
Detailed Photo
![]()
Packaging & Shipping
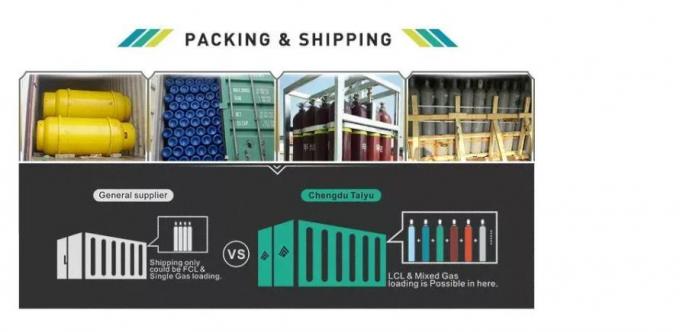



| MOQ: | 10kg |
| Price: | US $500/kg |
| Standard Packaging: | Cylinder/Tank |
| Delivery Period: | 30 days |
| Payment Method: | L/C, T/T |
| Supply Capacity: | 2000 Tons/Year |
Silicon tetrachloride (SiCl4) is a chemical compound composed of one silicon atom bonded to four chlorine atoms. It is a colorless liquid at room temperature, but it can also exist as a volatile gas. Here are some key points about silicon tetrachloride:
Chemical Composition: Silicon tetrachloride consists of one silicon (Si) atom bonded to four chlorine (Cl) atoms. Its chemical formula is SiCl4.
Properties: Silicon tetrachloride is a volatile liquid with a strong, pungent odor. It has a boiling point of 57.6 degrees Celsius (135.7 degrees Fahrenheit) and a melting point of -68.8 degrees Celsius (-91.8 degrees Fahrenheit). It is highly reactive and readily hydrolyzes in the presence of moisture, forming silicic acid and hydrogen chloride.
Production: Silicon tetrachloride is commonly produced through the reaction of silicon dioxide (SiO2) with carbon and chlorine gas:
SiO2 + 2C + 2Cl2 → SiCl4 + 2CO
This reaction occurs at high temperatures in the presence of a carbon-based reducing agent, such as carbon or coke.
Uses: Silicon tetrachloride has various industrial applications:
Silicon Production: It is a crucial precursor in the production of elemental silicon. Silicon tetrachloride is reacted with a reducing agent, such as magnesium, to obtain purified silicon in the form of crystalline or amorphous silicon.
Chemical Synthesis: Silicon tetrachloride is used as a starting material or intermediate in the synthesis of various silicon compounds, including silicones, silanes, and silicon carbide.
Optical Fiber Manufacturing: It is used in the production of high-purity silica glass, which is a key component in optical fibers for telecommunications.
Water Repellents: Silicon tetrachloride is used as a water-repellent treatment for fabrics, paper, and other materials.
Catalysts: It can be employed as a catalyst in certain chemical reactions, particularly in the production of polymers.
Safety Considerations: Silicon tetrachloride is toxic and corrosive. It can cause severe skin burns and eye damage upon contact. Inhalation of its vapors or fumes can be harmful to the respiratory system. Additionally, silicon tetrachloride reacts violently with water, releasing hydrogen chloride gas, which is highly corrosive and toxic. Proper safety precautions, such as the use of protective equipment and appropriate handling procedures, should be followed when working with silicon tetrachloride.
It's important to handle silicon tetrachloride with care and adhere to safety measures to mitigate potential risks associated with its corrosiveness and reactivity.
Basic Info.| Model No: | SiCl4 | Transport Package | ISO Tank |
| Specification | Standard | Trademark | CMC |
| Origin | Suzhou, China | HS Code | 2812190091 |
| Production Capacity | 1000t/Year |
Specification:
| CERTIFICATE OF ANALYSIS | |
| Product Name:Silicon Tetrachloride (STC) | |
| Inspection Item | Inspection Result |
| Product Appearance | Colorless Clear Liquid |
| HCL% | ------ |
| SiH2Cl2% | ------ |
| SiHCl3% | <0.1 |
| SiCl4% | >99.8 |
| High Boiling% | ------- |
| Al | <0.5ppm |
| Fe | <0.5ppm |
| Ti | <0.5ppm |
| Exogenous Impurities | ------ |
Detailed Photo
![]()
Packaging & Shipping



