


| MOQ: | 1kg |
| Price: | US $15/kg |
| Standard Packaging: | Cylinder/Tank |
| Delivery Period: | 15 days |
| Payment Method: | L/C, T/T |
| Supply Capacity: | 20000 Tons/Year |
Hydrogen fluoride (HF) is a chemical compound composed of hydrogen (H) and fluorine (F) atoms. It is an inorganic acid with a strong acidic nature. Here are some key points about hydrogen fluoride:
Properties: Hydrogen fluoride can exist as a colorless liquid or a colorless gas at room temperature, depending on the temperature and pressure. It has a boiling point of 19.5 degrees Celsius (67.1 degrees Fahrenheit) and a melting point of -83.6 degrees Celsius (-118.5 degrees Fahrenheit). HF is highly soluble in water and forms a strong acid when dissolved.
Production: Hydrogen fluoride is typically produced by the reaction of calcium fluoride (CaF2) with sulfuric acid (H2SO4). The reaction produces hydrogen fluoride gas, which can then be condensed into a liquid form. It can also be produced as a byproduct of certain industrial processes, such as the production of aluminum or phosphate fertilizers.
Uses: Hydrogen fluoride has various applications in different industries:
Chemical Industry: HF is used as a catalyst or reactant in several chemical processes, such as petroleum refining, production of fluorocarbons and fluoropolymers, and the synthesis of various organic and inorganic compounds.
Glass Etching: Hydrogen fluoride is commonly used for etching or engraving glass. It reacts with the silica (SiO2) in glass, creating a frosted or matte appearance on the surface.
Metal Surface Treatment: HF is utilized for cleaning and surface treatment of metals, particularly in industries such as electronics and semiconductor manufacturing. It can remove oxide layers and impurities from metal surfaces.
Laboratory Applications: Hydrogen fluoride is used in laboratories for various purposes, including sample preparation, glassware cleaning, and as an analytical reagent.
Safety Considerations: Hydrogen fluoride is a highly corrosive and toxic substance. It can cause severe burns to the skin, eyes, and respiratory system upon contact or inhalation. Inhalation of HF vapors or exposure to its solutions can be hazardous to human health. Proper safety precautions, including the use of appropriate protective equipment and ventilation, should be followed when handling hydrogen fluoride.
Due to its hazardous nature, the use, storage, and disposal of hydrogen fluoride should comply with strict safety protocols and regulations.
Basic Info.
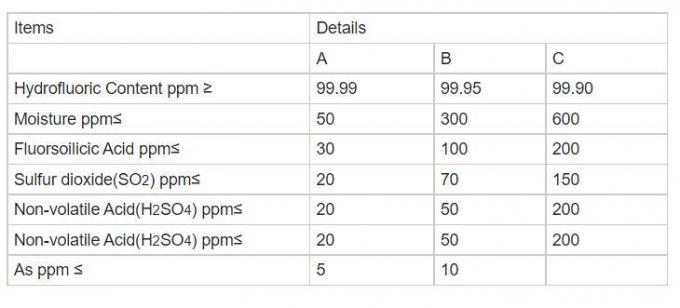
| Model No: | HF | Purity | 99.95%% |
| Transport Package | ISO Tank/Cylinder | Specification | ISO Tank/Cylinder |
| Appearance | CMC | Origin | Suzhou,China |
| HS Code | 2812190091 | Production Capacity | 30000 Metric Ton Per Year |
Specification:





| MOQ: | 1kg |
| Price: | US $15/kg |
| Standard Packaging: | Cylinder/Tank |
| Delivery Period: | 15 days |
| Payment Method: | L/C, T/T |
| Supply Capacity: | 20000 Tons/Year |
Hydrogen fluoride (HF) is a chemical compound composed of hydrogen (H) and fluorine (F) atoms. It is an inorganic acid with a strong acidic nature. Here are some key points about hydrogen fluoride:
Properties: Hydrogen fluoride can exist as a colorless liquid or a colorless gas at room temperature, depending on the temperature and pressure. It has a boiling point of 19.5 degrees Celsius (67.1 degrees Fahrenheit) and a melting point of -83.6 degrees Celsius (-118.5 degrees Fahrenheit). HF is highly soluble in water and forms a strong acid when dissolved.
Production: Hydrogen fluoride is typically produced by the reaction of calcium fluoride (CaF2) with sulfuric acid (H2SO4). The reaction produces hydrogen fluoride gas, which can then be condensed into a liquid form. It can also be produced as a byproduct of certain industrial processes, such as the production of aluminum or phosphate fertilizers.
Uses: Hydrogen fluoride has various applications in different industries:
Chemical Industry: HF is used as a catalyst or reactant in several chemical processes, such as petroleum refining, production of fluorocarbons and fluoropolymers, and the synthesis of various organic and inorganic compounds.
Glass Etching: Hydrogen fluoride is commonly used for etching or engraving glass. It reacts with the silica (SiO2) in glass, creating a frosted or matte appearance on the surface.
Metal Surface Treatment: HF is utilized for cleaning and surface treatment of metals, particularly in industries such as electronics and semiconductor manufacturing. It can remove oxide layers and impurities from metal surfaces.
Laboratory Applications: Hydrogen fluoride is used in laboratories for various purposes, including sample preparation, glassware cleaning, and as an analytical reagent.
Safety Considerations: Hydrogen fluoride is a highly corrosive and toxic substance. It can cause severe burns to the skin, eyes, and respiratory system upon contact or inhalation. Inhalation of HF vapors or exposure to its solutions can be hazardous to human health. Proper safety precautions, including the use of appropriate protective equipment and ventilation, should be followed when handling hydrogen fluoride.
Due to its hazardous nature, the use, storage, and disposal of hydrogen fluoride should comply with strict safety protocols and regulations.
Basic Info.
| Model No: | HF | Purity | 99.95%% |
| Transport Package | ISO Tank/Cylinder | Specification | ISO Tank/Cylinder |
| Appearance | CMC | Origin | Suzhou,China |
| HS Code | 2812190091 | Production Capacity | 30000 Metric Ton Per Year |
Specification:




